Home > New Product Labeling due to MDR
New Product Labeling
New Product Labeling due to MDR
According to the new regulation (EU) 2017/745 our product labelling will be adapted in accordance with the new legal requirements.
Below figure shows a sample of the new product label for a medical device:
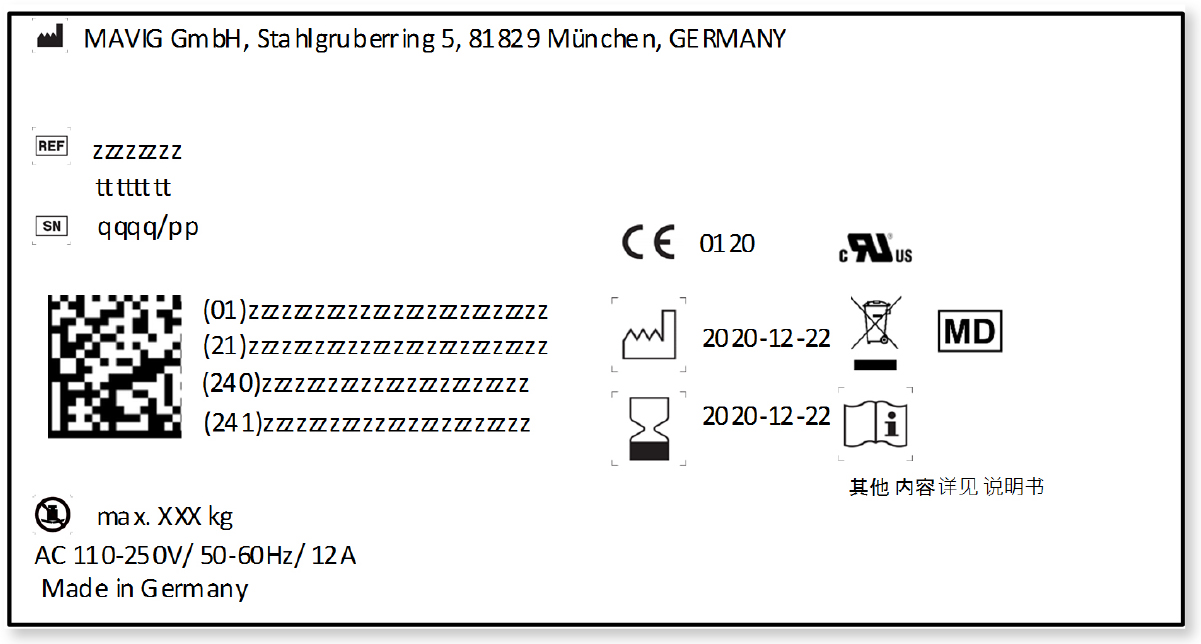
The transport label will be updated correspondingly.
Starting from May 2021, all our technical products will comply with the new standard.
Click here to access the published MDR on the Official Journal of the European Union.



